Reproductive & Hormonal Factors
The magnitude of the associations of reproductive factors with cancer risk is relatively small. However, these factors affect all women; therefore, they have a large impact at the population level.
Reproductive patterns and exposure to reproductive hormones play a role in the development of some cancers in women. Economic, political and societal shifts in the last century have been marked by profound changes in sexual maturation and reproductive patterns. These changes have led to increased lifetime number of monthly menstrual cycles, which is associated with higher risk of breast, endometrial and ovarian cancers. Although not fully understood, one mechanism that could underlie these relationships is increased exposure to endogenous estrogen and progesterone levels. Other aspects of menses may play a role in the development of some types of ovarian cancers. Longer-term breastfeeding (Map & Figure 1) lowers risk of most types of breast cancer, likely through cessation of the menstrual cycle, changes to the hormonal milieu, and profound cellular changes to the breast tissue.
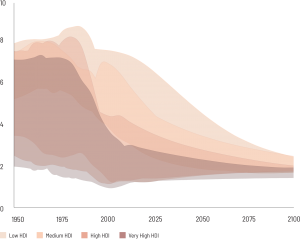
While shifting patterns of reproductive factors, such as decreasing age at menarche, increasing age at first birth, and fewer births per woman, continue in many developing countries—and may have contributed to increases in incidence rates for hormone-related cancers—these trends have plateaued in many developed countries. (Map & Figure 2)
Average number of births per woman is a maximum of 8 in 1950 in representative low-HDI countries and is projected to decline to around 2 in 2100. Average number of births per woman is between 6 and 8 in 1950 in representative medium-HDI countries and is projected to decline to around 2 in 2100. Average number of births per woman is between 4 and 8 in 1950 in representative high-HDI countries and is projected to decline to around 2 in 2100. Average number of births per woman is between 2 and 7 in 1950 in representative very high-HDI countries and is projected to decline to around 2 in 2100.
In addition, many women in higher-income counties are exposed to sustained use of exogenous hormones for contraception, reproductive assistance, and menopausal symptoms. Hormonal contraceptive users have a slight, transient increase in the risk of breast cancer, but a moderate and long-term reduction in the risk of some types of ovarian cancer and endometrial cancer. (Figure 3)
Although use of fertility drugs is a relatively recent exposure, early studies indicate that use of these powerful hormones does not increase cancer risk. Menopausal hormone therapy increases risk of breast and endometrial cancer dependent on formulation, timing of use, and body size, but may be associated with a decreased risk of colorectal cancer.
Breastfeeding duration:
Victora CG, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490.
Text:
Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 2015;99(Pt A):8–10.
Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status–a systematic review and meta-analysis. Ann Oncol. 2015;26(12):2398–2407.
Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580 e581–580 e589.
Murphy N, Ward HA, Jenab M, et al. Heterogeneity of colorectal cancer risk factors by anatomical subsite in 10 European Countries: A multinational cohort study. Clin Gastroenterol Hepatol. 2018.
Williams CL, Jones ME, Swerdlow AJ, et al. Risks of ovarian, breast, and corpus uteri cancer in women treated with assisted reproductive technology in Great Britain, 1991-2010: data linkage study including 2.2 million person years of observation. BMJ. 2018;362:k2644.
Map 1 and Figure 1:
Victora CG, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017): 475–490.
Quigley MA, Carson C. Breastfeeding in the 21st century. Lancet. 2016;387(10033): 2087–2088.
Map 2:
United Nations, Department of Economic and Social Affairs, Population Division (2017). World Population Prospects: The 2017 Revision, custom data acquired via website.
Figure 2:
United Nations, Department of Economic and Social Affairs, Population Division (2017). World Population Prospects: The 2017 Revision, custom data acquired via website.
Figure 3:
Note: Etiologic heterogeneity is an active area of research for most of these cancers. For example, there is active research into the disparate role of parity in the etiology of estrogen receptor positive compared to triple negative breast cancer. The table considers the hormonal and reproductive risk factors in association to risk of the cancer site overall.
Evidence is based more strongly on studies with prospective exposure assessment.
An N. Oral Contraceptives Use and Liver Cancer Risk: A Dose-Response Meta-Analysis of Observational Studies. Medicine. 2015;94(43):e1619.
Appleby P, Beral V, et al. for the International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370(9599):1609–1621.
Ben Khedher S, Neri M, Papadopoulos A, et al. Menstrual and reproductive factors and lung cancer risk: A pooled analysis from the international lung cancer consortium. Int J Cancer. 2017;141(2):309–323.
Brinton LA, Felix AS. Menopausal hormone therapy and risk of endometrial cancer. Journal Steroid Biochem Molecular Biol. 2014;142:83–89.
Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 2015;99(Pt A):8–10.
Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(1):20–38.
Chlebowski RT, Anderson GL, Sarto GE, et al. Continuous Combined Estrogen Plus Progestin and Endometrial Cancer: The Women’s Health Initiative Randomized Trial. J Natl Cancer Inst. 2016;108(3).
Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374(9697):1243–1251.
Costas L, de Sanjose S, Infante-Rivard C. Reproductive factors and non-Hodgkin lymphoma: a systematic review. Crit Rev Oncol Hematol. 2014;92(3):181–193.
Cote ML, Alhajj T, Ruterbusch JJ, et al. Risk factors for endometrial cancer in black and white women: a pooled analysis from the Epidemiology of Endometrial Cancer Consortium (E2C2). Cancer Causes Control. 2015;26(2):287–296.
Gaudet MM, Gapstur SM, Sun J, Teras LR, Campbell PT, Patel AV. Oophorectomy and hysterectomy and cancer incidence in the Cancer Prevention Study-II Nutrition Cohort. Obstet Gynecol. 2014;123(6):1247–1255.
Gaudet MM, Gierach GL, Carter BD, et al. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res. 2018;78(20):6011–6021.
Green J, Roddam A, Pirie K, et al. Reproductive factors and risk of oesophageal and gastric cancer in the Million Women Study cohort. Br J Cancer. 2012;106(1):210–216.
International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006;119(5):1108–1124.
Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580 e581–580 e589.
LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–1314.
Ma X, Zhao LG, Sun JW, et al. Association between breastfeeding and risk of endometrial cancer: a meta-analysis of epidemiological studies. Eur J Cancer Prev. 2018;27(2):144–151.
Murphy N, Strickler HD, Stanczyk FZ, et al. A Prospective Evaluation of Endogenous Sex Hormone Levels and Colorectal Cancer Risk in Postmenopausal Women. J Natl Cancer Inst. 2015;107(10).
Murphy N, Ward HA, Jenab M, et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clinl Gastroenterol Hepatol. 2019;17(7):1323–1331 e1326.
Roura E, Travier N, Waterboer T, et al. The Influence of Hormonal Factors on the Risk of Developing Cervical Cancer and Pre-Cancer: Results from the EPIC Cohort. PloS one. 2016;11(1):e0147029.
Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 013;31(20):2607–2618.
Tsilidis KK, Allen NE, Key TJ, et al. Oral contraceptives, reproductive history and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2010;103(11):1755–1759.
Zhong GC, Liu Y, Chen N, et al. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: a systematic review and dose-response meta-analysis of observational studies. Hum Reprod Update. 2016;23(1):126–138.
Explore Related Topics
This figure cannot be displayed at mobile resolutions.
To view this figure, please visit the desktop version of this website or download the PDF file of the book chapter.